Different Types of Quality Management Systems

The renowned art critic John Ruskin’s insight resonates across industries, highlighting the essence of deliberate effort and meticulous planning in achieving and sustaining quality, be it in art or business. In today’s fiercely competitive landscape, an organization’s commitment to consistently upholding high-quality procedures and products significantly influences its prospects for success.
Quality, as Ruskin aptly observed, isn’t a happenstance occurrence but a deliberate pursuit. It necessitates continual efforts, thorough analysis, and a dedicated commitment to improvement.
Enhancing your Quality Management System (QMS) stands as one of the most valuable investments an organization can make. Strengthening this system serves as the bedrock for delivering top-notch products and services while fostering a culture of quality excellence throughout the organization.
In essence, a robust and evolving Quality Management Software not only ensures the delivery of superior products and services but also fosters a competitive edge, customer satisfaction, and long-term success in today’s dynamic business landscape.
A Quality Management System is Beneficial for a Variety of Reasons.
A Quality Management System (QMS) refers to a structured framework of policies, processes, procedures, and resources established within an organization to ensure it consistently meets and exceeds quality standards in its products or services.
When discussing different types of QMS, it’s essential to recognize that various standards or methodologies exist, each offering a unique approach to achieving and maintaining quality. These standards aim to guide organizations in implementing effective quality management practices.
Some Notable QMS Standards and Methodologies Include:
- ISO 9001: One of the most recognized international standards for QMS, emphasizing a process-oriented approach to quality management, customer satisfaction, and continuous improvement.
- Six Sigma: A methodology focused on reducing defects and variability in processes by using statistical methods to achieve near-perfect results, often aiming for no more than 3.4 defects per million opportunities.
- Total Quality Management (TQM): A holistic approach that involves all employees in continuous improvement to meet or exceed customer expectations. TQM emphasizes customer focus, continuous improvement, and employee involvement.
- Lean Manufacturing: A methodology primarily focused on minimizing waste and maximizing value through continuous improvement efforts, streamlining processes, and optimizing efficiency.
- Capability Maturity Model Integration (CMMI): Primarily used in software and systems engineering, CMMI provides guidelines for improving processes and capabilities within an organization.
Understanding these different methodologies allows businesses to assess and choose the QMS that aligns best with their goals, industry requirements, and organizational culture. Each approach has its unique strengths and focuses, catering to various business needs and objectives.
What is Quality Management Software?
Quality Management Software facilitates the coordination of processes, policies, procedures, documentation, and resources essential for meeting customer needs and expectations.
As industrial manufacturing expanded, Quality Management Systems emerged to ensure consistent outcomes amid higher volumes and increased specialization.
Today’s market demands precision, speed, and scalability, necessitating Quality Management Software to reduce errors, minimize delays, and enhance customer satisfaction. Notably, the concept of quality has broadened to encompass environmental and privacy concerns alongside production standards.
Modern QMS requires collaboration among people and efficient allocation of capital and resources. This underscores the need for dedicated QMS system.
For implementing a QMS or achieving specific quality compliance, choosing a suitable Quality Management Software from the options listed below is vital.
Types of Quality Management System
The ISO systems stand out as the most prevalent and referenced types of quality management solutions and standards.
The ISO family of quality standards finds application across diverse industries, addressing both quality and sustainability. While ISO 9001 stands as the globally recognized and widely adopted standard, specific industries may benefit from additional accreditations and standards tailored to their requirements.
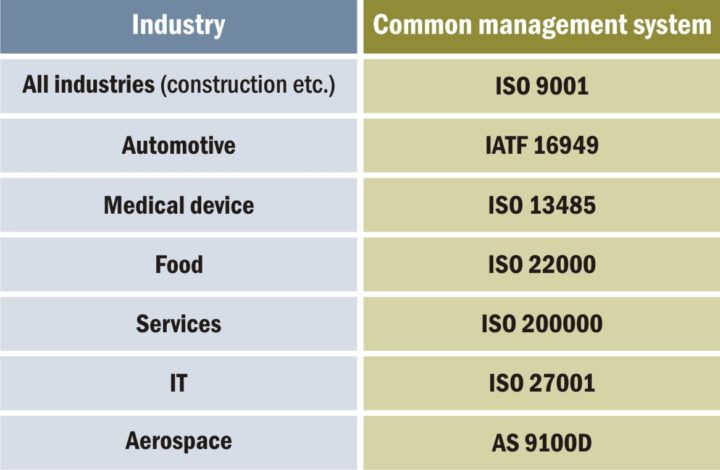
Despite varying quality levels across industries, they often share common principles and employ similar quality statements, as illustrated above.
While many companies prioritize meeting these quality standards and management systems, efficient coordination and management of these components become crucial, particularly in complex operational scenarios. This is where the ‘types of quality management software‘ play a pivotal role.
Key Takeaway
Implementing a QMS system is vital for embedding quality across a company’s operations and products. The choice of system depends on the company’s specific quality criteria, with ISO 9001 being among the most prevalent standards.
Build a robust QMS for your company today and set yourself apart in a competitive landscape. Contact us now to get started!






