Four Key Ways QMS Software Supports Change Management

- Overview
- What Is Change Management?
- Process of Change Management
- How QMS supports Change Management
- Conclusion
Overview
Heightened global competition has motivated manufacturing companies to constantly innovate their product offerings. To keep pace with this accelerated pace of change, companies need to develop a dynamic work culture and environment. Change does not come naturally to people or organizations and without proper management, a change can cost precious time and money. It is also vital to measure the scale, magnitude, duration and strategic importance of the change.
Organizations would do well to address and balance the multiple aspects of change like people, process, technology etc. Creating a change management plan can help with a smoother transition from the old to the new. While proactively combating resistance to change, companies need to set clear goals for the changes and monitor the results vigorously. By creating effective strategies to implement change, manufacturing organizations can ease out the process for their people.
What is Change Management?
Change management is the process of planning, implementing and validating changes in the organization. It refers to the organization’s ability to handle modifications like implementing new technology, adjusting existing processes and shifting organizational hierarchy. Change managers need to set key performance indicators (KPIs) and relevant metrics to measure the effects of the change. In the present scenario of rapid product changes, the co-ordination of design centers, suppliers, testing labs, manufacturing facilities, offshore plants and the marketing department becomes very critical in change management.
Change can be a consequence of innovation, customer complaints, material returns, non-conformances and similar occurrences. Upon implementing a QMS (Quality Management System) in the organization, managers can standardize their response to every new development with automation. It can even shorten the change process cycle time and reduce the impact of change on everyday operations. We will elaborate about the benefits of QMS for change management further in the blog, but let us first discuss the process of change management.
Process of Change Management
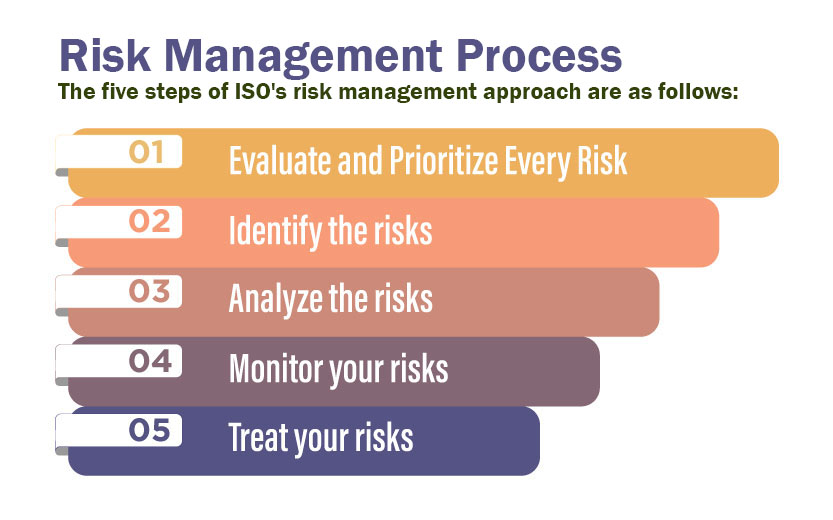
Businesses must constantly evolve and adapt to meet a variety of challenges like changes in technology, rise of new competitors, a shift in laws/regulations or any underlying economic trends. Change processes have a set of starting conditions and a functional endpoint. Change initiatives may be different from each other, but they typically follow a similar process. To effectively manage change, managers must thoroughly understand the steps involved. The key steps in the change management process are listed below:
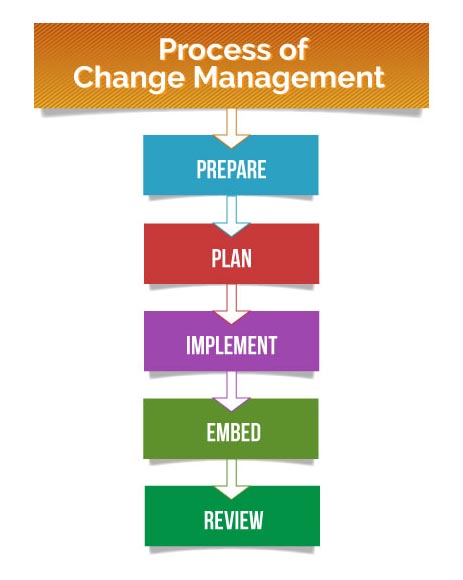
- Prepare – The organization needs to be prepared for change, both logistically and culturally. All employees should recognize and understand the purpose of change.
- Plan – The definitions of KPIs and strategic goals should be pre-defined. The scope of the project and the hierarchy of reporting must be laid out beforehand.
- Implement – The steps laid out by the management must be diligently followed. Employees need to anticipate roadblocks and prevent, remove, or mitigate them once identified.
- Embed – The changes inculcated by the process should become part of the company culture. Change managers need to ensure that the process doesn’t revert back to the old ways.
- Review – conducting analysis and review to make sure that the change was successfully implemented. It can offer valuable insights that can be leveraged in future change efforts.
How QMS Supports Change Management
Paper-based quality processes can hinder innovation-centric changes and even cause non-compliance in a regulated industry. By implementing a QMS, the change is managed in a workflow-based system and quality is benchmarked at every step. The “Make it once, change if often” philosophy of the present product developers can benefit from the dynamic work environment created with the help of a QMS. Let’s discuss the four key ways QMS software supports change management:
- Change Process Automation – Automation of the change management process results in increased control over the variables. A QMS provides the right tools for the organization to exercise control over the tasks, processes and documents of change. Everything including routing, follow-up and approval can automated through a QMS. Managers can effectively define process ownership and user control for increased accountability. Automatic escalation can speed up departmental collaboration and new approvals can automatically supersede any old versions.
- Transparent Change History – A time-stamped and secure audit trail enables the stakeholders/auditors/regulators to view the entire edit history of documents in a chronological order. Complete record of people who request, approve and validate the changes is also saved in the system to allow for complete transparency. QMS can also present key metrics relating to open change tasks on a unified dashboard and generate reports in an efficient manner to identify bottlenecks and other issues.
- Closed-loop Change Management – Vigilance is not enough for compliance. Organizations need a QMS to follow a proactive approach by connecting all quality processes. CAPA issues and deviations will seamlessly trigger change management and training through the QMS system. If a CAPA issue requires new training, the QMS will facilitate task completion with automatic notification and follow up. The system unites different processes and individual users into a holistic compliance effort so that every issue is identified and resolved.
- Shorter Change Cycle Time – Efficient change management and change control leads to timely regulatory submissions and faster time to market. Once a change control request gets approved, a QMS automatically generates and routes all the related documents (Ex. SOP, change order) to the concerned departments. Gone are the days, when this process was done via long trails of e-mail or through in-person meetings. Every step of implementation and post-implementation assessment are automated with QMS to close the loop quickly and effectively.
QMS can help companies achieve change management and change control according to cGMP regulations and ensure product safety and quality. While every regulated company wants to comply with requirements, they need to leverage the right QMS software to attain and maintain compliance despite the constant change in their operations. QualityPro is a next-gen QMS software that supports change management along with all the other quality management requirements of a manufacturing company. If you wish to request a demo, please click here.