
In the biotechnology industry, innovation drives advancements at a breakneck pace, rapidly transforming the healthcare industry, among others, for the better. However, this growth has perennially come with increased challenges and complexities. And how exactly can the biotechnology industry cope with them in the modern times?
Read this blog to figure out how you can choose the best QMS software for your biotechnology business to overcome complexities and ensure success.
Overview
Innovation is one thing you would always associate with the biotechnology industry. In fact, it is this innovative side of the industry that has played a major role in helping it expand to reach to the heights it has, today. However, it is not innovation alone that has driven this industry.
If innovation is important, so is precision. When you talk about biotechnology industry, you are talking about sensitive research in the areas of new drug development, improved agricultural practices, and regulatory and environmental stability, among others.
Add the pressure to reduce time to market, deliver safe & effective products, and address the lack of cross-functional visibility, and you have a whole lot of challenges and growth-related complexities on your hands.
In such scenario, quality management isn’t just a choice for biotechnology businesses. It’s a necessity.
A biotechnology quality management system, or a QMS software tailored for the biotech industry can help the businesses with majority of the things, if not all, mentioned above. For starters, it can help the biotech manufacturing businesses build a culture of quality across their organization, which in turn can address most challenges and complexities.
To understand this, let’s consider the scenario of a biotech organization which is operating without a QMS. Let’s see what challenges they face in the absence of such system, and how its presence can make them a better operator.
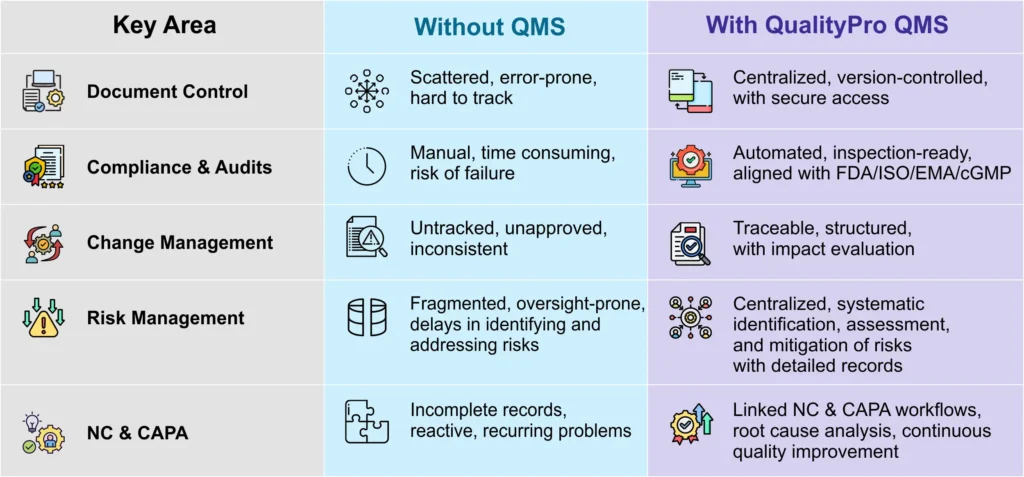
Data and Document Chaos
Critical documents such as the Standard Operating Procedures (SOPs), manufacturing batch records, training logs, etc. are scattered across various platforms or spreadsheets, in the absence of a dedicated QMS with document management system (DMS) in the biotech industry. This can lead to serious operational inefficiencies such as errors lost time, and even compliance challenges.
These issues can be effectively addressed with a QMS which comes with integrated DMS. Such a system can streamline their operations by offering all the stakeholders access to a secure and centralized platform where they can manage the SOPs and other critical documents, and get up-to-date information.
Among other things, it offers electronic document control with version history, and role-based access, which eventually facilitates compliance, reduces redundancies and errors, improves traceability, and enhances the organizational productivity.
Regulatory Non-Compliance and Audit & Inspection Inefficiency
It is a no-brainer that industries such as biotech are heavily regulated due to the critical safety and efficacy concerns tied to their products and processes. And in such industries, adherence to strict standards such as the FDA (Food and Drug Administration), ISO (International Organization for Standardization), and EMA (European Medicines Agency), among others, is critical. But, in the absence of an effective QMS, biotech organizations struggle to meet these stringent requirements, which leads to serious risks such as failed audits and product recalls.
However, a good QMS system, with its automated workflows and audit trails aligned with FDA, ISO and other standards, streamlines compliance and ensures that the biotech manufacturing organization is always inspection-ready. Also, through its inspection management feature, it offers organized checklists, templates, and inspection plans to standardize QC tests and expected outcomes.
Together, they create a robust foundation for audit preparation, supporting all types of audits, and thus helping biotech organization in confirming adherence to set standards and assuring ongoing quality enhancement.
Disabled Change Management
In the absence of a quality management system software for biotechnology with change management functionality, biotech companies often encounter significant challenges in managing change. They fail to record change requests arising from different sources, say audit findings for example. This makes it difficult to trace their origin and/or evaluate their impact.
Additionally, this lack of traceability makes the process of tracking lifecycle of changes right from their initiation to implementation, error-prone and cumbersome.
However, a good biotech QMS software can help address these issues with its dedicated change management function. Furthermore, it can offer a systematic approach to change management, facilitating process modifications initiated due to changes, while ensuring the change gets communicated across teams in a consistent manner so that there are no quality issues or uneven practices.
Unregulated Risk Management
In the absence of a QMS software, biotechnology companies suffer with a fragmented risk management process, which is severely prone to oversight and inefficiency. The companies find it extremely difficult to identify, analyse, and address different types of risks, such as non-compliance or safety concerns, in the absence of a centralised system. This delay in identification of potential hazards increases the likelihood of regulatory penalties or compromised product quality.
But a sound QMS for biotech companies with risk management module provides tools to systematically identify, assess and mitigate risks while maintaining detailed records. Its centralized platform enables proactive monitoring of potential issues by offering a consolidated view of critical risk information, empowering the biotech companies to assure their stakeholders that regulatory compliance is maintained, and legal complications are avoided, at all times.
Ineffective Issue Resolution
In the absence of a QMS system, biotech businesses struggle to efficiently record the events of non-conformances (NCs), or address the recurring issues. Incomplete or incorrect documentation and disorganized workflows prevent them from making corrective and preventive actions (CAPAs), which in turn prevent them from meeting regulatory standards or achieving other operational objectives.
A good QMS software for biotechnology industry with NC and CAPA management feature streamlines quality issue resolution and ensures regulatory compliance. While NC management automatically logs non-conformance events and links them to the root causes, CAPA management uses structured workflows to document and track corrective actions with approvals and evidence. These features help biotech businesses such as pharma businesses prevent recurring issues, reduce risks, and maintain compliance.
How to Choose the Best Quality Management Software for Biotechnology?
If you have stayed with us this far, you likely have a fair idea about what to look for in your prospective biotechnology QMS software, which is best suited for your biotech business. The capabilities discussed earlier, along with some additional features such as Training Management and Complaint Management, comprehensively address the quality management needs of your biotech manufacturing operations.
Additionally, choosing the right QMS for biotech companies requires careful consideration of other few key criteria. You must look for a system that’s tailored for your biotech workflows, and not one that’s adapted from another industry. It should be scalable, must offer ease of use, and should be from a vendor that provides robust support, and training, to maximize your investment.
QualityPro by TecWork: Best QMS Software for Biotechnology Industry
This brings us to the concluding part of this blog, where we reveal the best QMS for your business in biotechnology industry. Hands down, it has to be QualityPro by TecWork Global Business Solutions.
QualityPro comes loaded with everything mentioned above, and more, providing your business with a competitive edge and a strategic advantage that drive innovation and success. A modern and feature-rich solution, it not just covers your business’ quality management needs for today, but future-proofs your business for the needs of tomorrow as well.
Ready to take the next step? Feel free to connect with our sales team to schedule a demo and learn more about how our QMS software can benefit your organization.





