Why Automated Audit Management System is Important?

- Overview
- What is an audit? Why is it necessary?
- Importance of automated audit management system
- Conclusion
Overview
Before starting a blog about audit management, it is important to establish that the purpose of an audit is not to assign blame, but to find a solution. Many business owners and management personnel view audits as a necessary evil, a box to be ticked to satisfy compliance. However, in reality, an audit can add real value to your company. It can help you spot weaknesses in your infrastructure, expose non-conforming behavior and refine your business processes.
An audit ensures that all the steps of a manufacturing operation are being performed as intended. Manufacturing process audits should verify that procedures are properly followed, problems are quickly corrected, consistency of process is maintained, and there is continuous improvement and corrective action as needed. Companies can outperform their competition with the ability to take quick action on quality data gained from these audits and modify customer outcomes immediately.
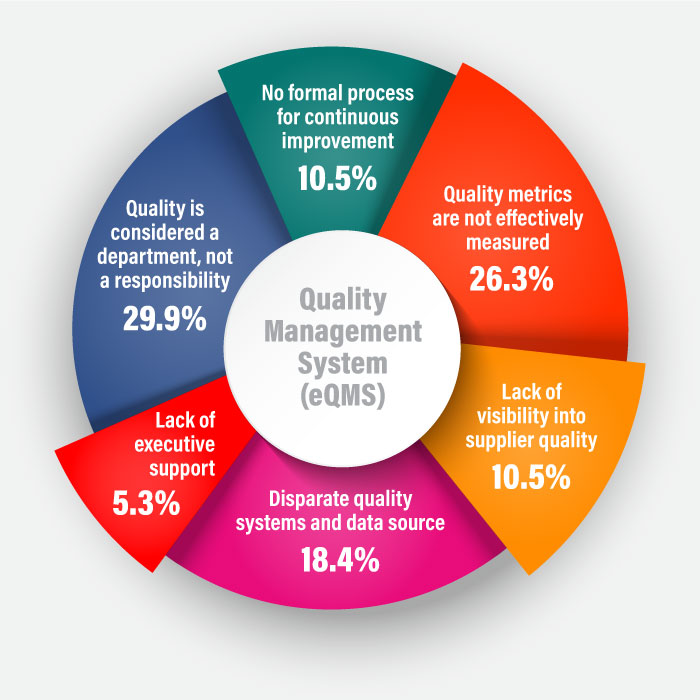
Historically, the audit process was conducted manually with paper-based checklists. However, manual audits are no longer suitable in today’s digital age. Technology-enabled tools have taken over the responsibility of managing audit activities to ensure consistent quality and compliance. Regulated companies are increasingly using automated audit management systems to maximize effectiveness while managing costs and streamlining their audit process without sacrificing flexibility.
What is an audit? Why is it necessary?
An audit is the objective analysis and examination of a particular business operation to evaluate its conformity with the expected standards. Audits can have different purposes. A financial audit conducted to check the accuracy of the company’s financial records. Compliance audits are designed to ensure that the company is obeying the latest ISO 9001 standards. A quality audit checks for the application of predefined quality standards. These and many such audits can either be internal or external.
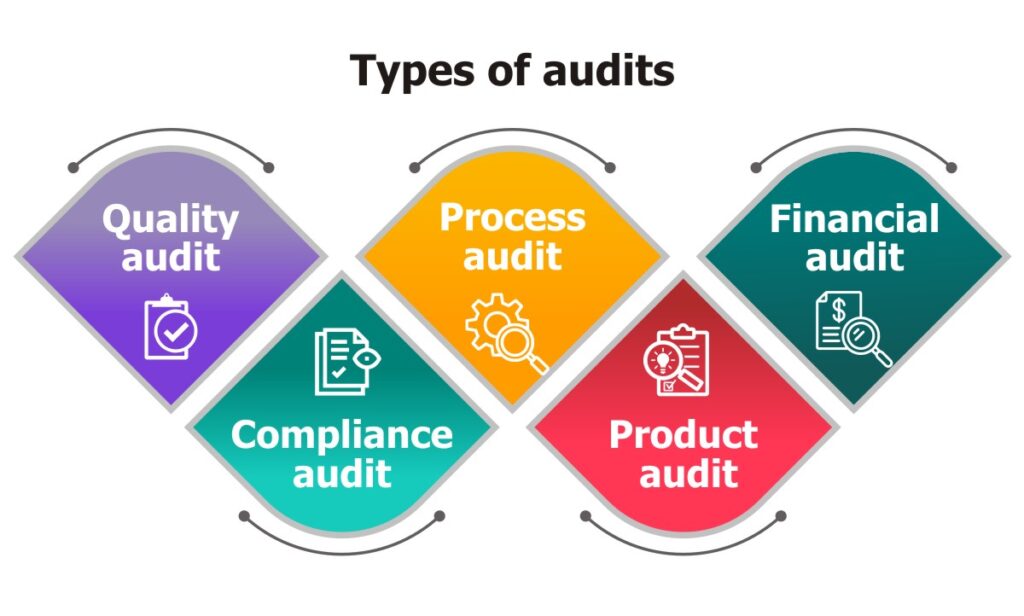
The procedure will depend on the type of audit being performed and the standards governing the auditor’s work. Audits are necessary as they can help the manufacturer pinpoint areas that need improvement. They offer a reliable assessment of the manufacturer’s capability and facilities and provide an overview of the company’s processes, operations, and workflows. Moreover, audits are valuable tools used to uphold employee safety, evaluate operational efficacy and uncover non-conformance issues.
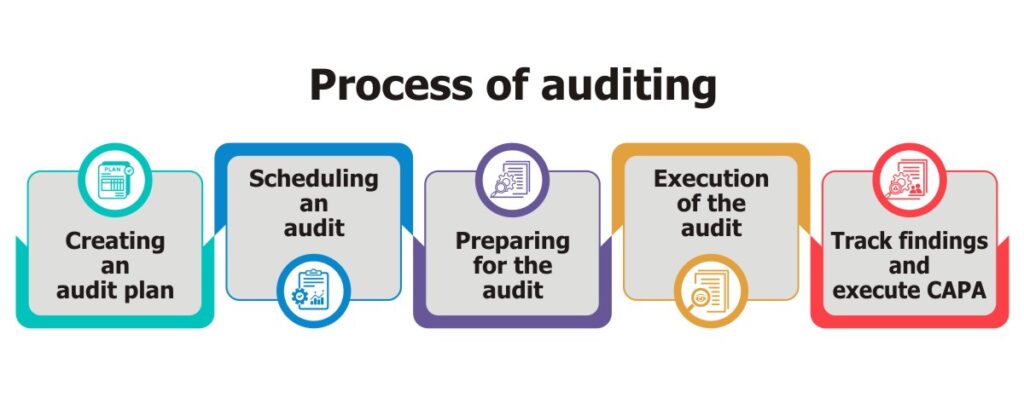
Importance of automated audit management systems
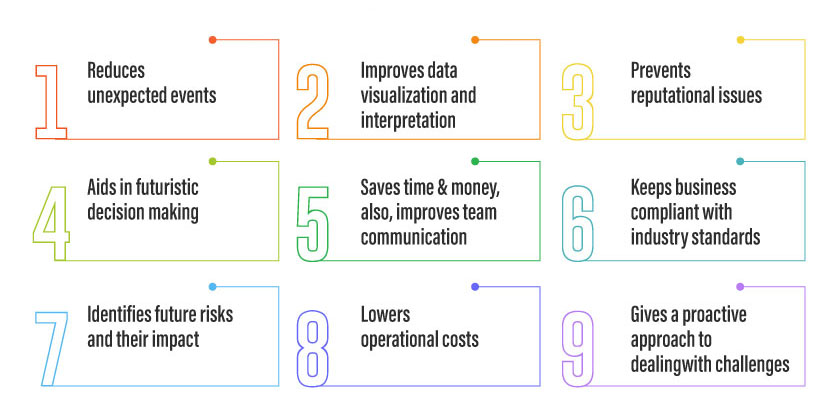
Technology aids in drastically reducing the cost of audits. Centralized operations and simplified reporting allow for accurate, actionable insights, so that auditors don’t have to waste time tracking down information in spreadsheets. An effective audit management system simplifies the day-to-day work by standardizing methods and automating repetitive tasks, hence boosting the performance and effectiveness of the entire auditing process.
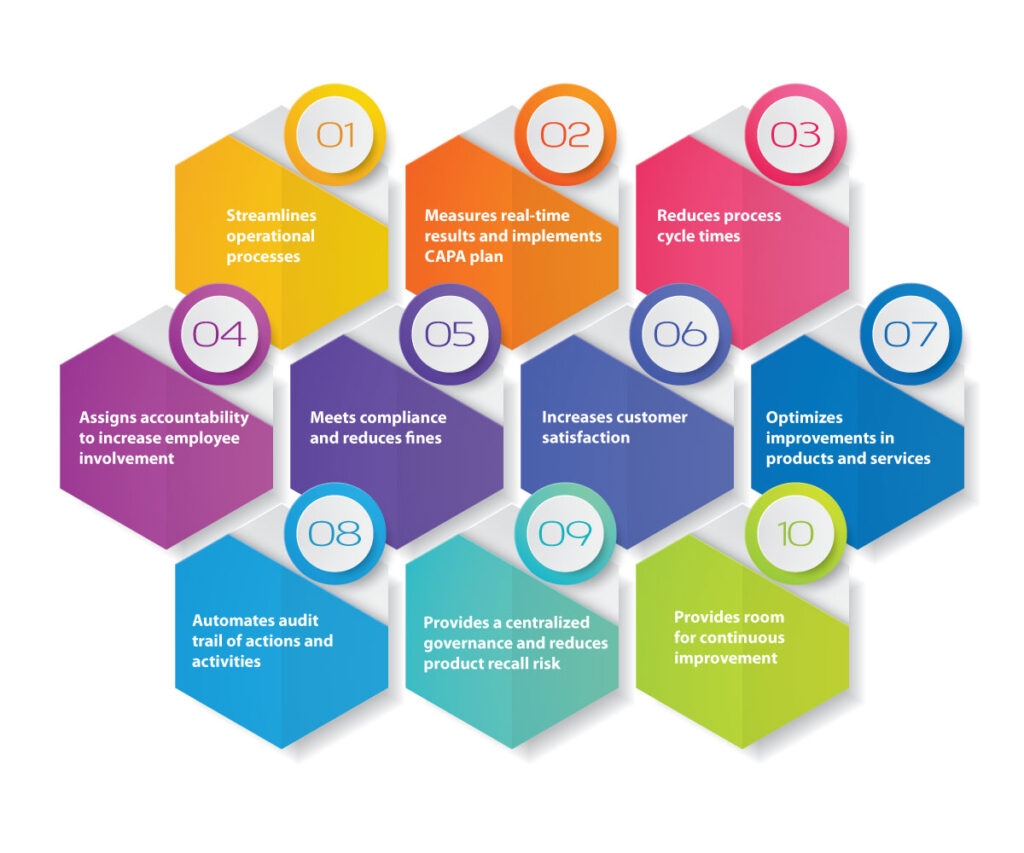
An audit management software can plan, schedule, perform, report and close-out the process with automated workflows. It helps companies improve business performance by reducing risk and managing compliance. It also provides end-to-end functionality for the entirety of the audit life cycle which includes audit planning, checklists, data collection, audit reports and CAPA recommendations.
The key benefits of an automated audit management system are listed below:
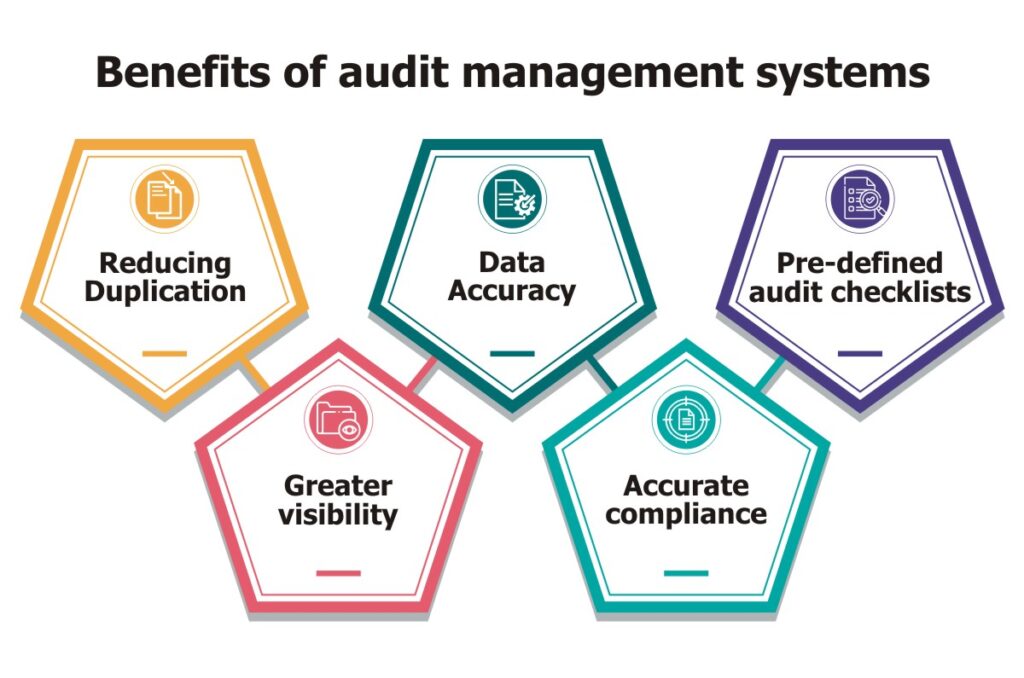
- Reducing Duplication – Conducting an on-site audit coupled with filling piles of duplicate data can be frustrating for the auditor. Using an audit management software, the auditor can gather evidence and answer questionnaires on the spot. With anytime & anywhere access, this software allows you to download the audit and attach relevant data as it is gathered. As a result, there is no duplication of effort, saving precious company hours or even days for every audit. The customizable report feature makes it easy to display data visually.
- Data Accuracy – Writing notes or completing a paper-based checklist can be error-prone and bias driven. With an audit management system, auditors can capture real-time evidence and attach any type of common file including videos, photos and sound recordings. They can attach evidence of non-conformances that can be viewed by everyone in the same way, leaving no room for misinterpretation. The ability to collect data from different sources and applications reduces operational oversights and ensures a smarter decision-making system.
- Pre-defined audit checklists – With an audit management system, companies can get pre-defined audit checklists. Audit managers can configure and add new fields in the checklist to suit business requirements. Standardization of processes and documents ensure the implementation of industry best practices. The checklist includes gathering preliminary information to scope the audit, determining the key business risks, identifying areas for more audit attention and informing managers about data needs.
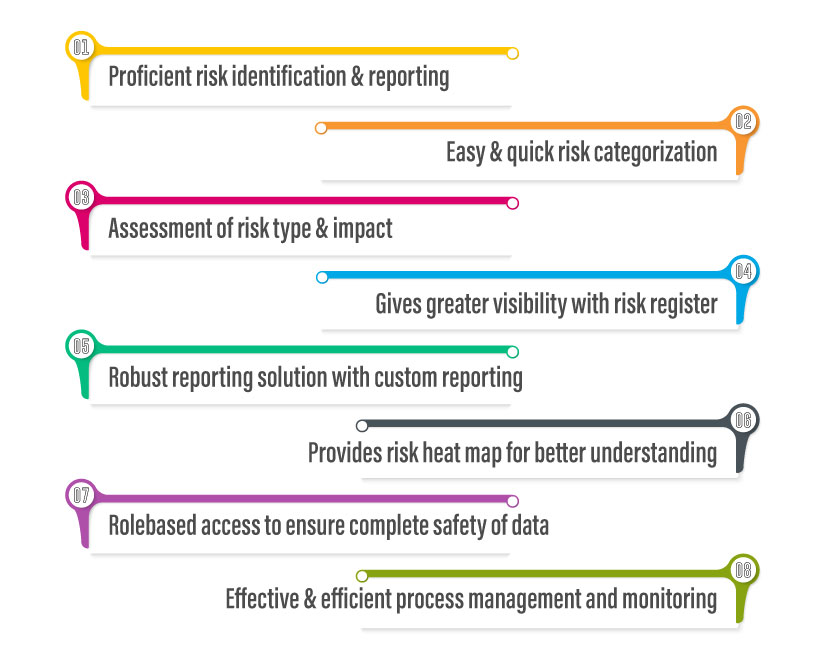
- Greater visibility – Audit management software has powerful reporting capabilities to provide greater visibility into critical audit results like findings, best practices & auditor recommendations. It also facilitates quick generation of detailed audit reports and distribute them to all the involved parties. Auditors can produce different types of reports for different types of audits, each providing you valuable insights. A unified dashboard of audit reports and results helps you make informed business decisions on the required improvements and changes.
- Accurate compliance – Working from paper trails makes compliance difficult to prove – one missing file and you risk non-conformances or failing the audit completely. Audit management systems allow you to easily plan and schedule regular internal audits, with automated workflows triggered when a finding is raised. This makes it very easy to demonstrate commitment to regulations and other quality standards, delivering a full and comprehensive audit trail for anyone investigating your compliance.
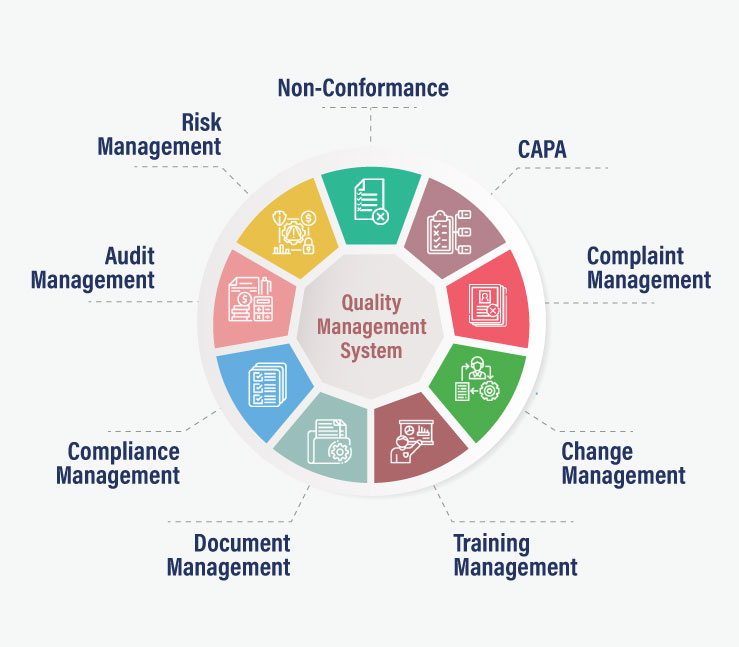
Audit management solutions help enterprises align their critical processes with business quality objectives while mitigating operational challenges. They empower organizations by giving the more control and confidence in addressing compliance, quality, and operational risks efficiently through regular audits. One such software, QualityPro prepares you to succeed with a next-generation approach to identify and eliminate inconsistencies throughout the quality system, products, and processes. If you want to automate your quality management activities with QualityPro, click here.