
Quality audits are very crucial in checking compliance with regulatory requirements in any industry. In a pharmaceutical setup, these audits check whether the complex processes and procedures of your pharmaceutical unit follow the set standard.
For examples, let us assume that your pharmaceutical company is making paracetamol to sell in the market. It is only through quality audits that your consumers, and regulatory bodies will have assurance that quality of your product meets the standard and therefore they are safe and effective to use.
Quality audits are very complex and time taking process. But if you know how to prepare for quality audits, and how to be prepared for an audit, most of your worries will go away.
So, in this blog we will discuss all that you need to know regarding quality audit in pharmaceutical industry and will look into the ways to prepare for your next or probably the first audit.
- What is Pharmaceutical Quality Audit?
- Types of Audits in Pharmaceutical Industry
- Ways in Which Pharmaceutical Audits are Conducted
- How to Prepare for a Pharmaceutical Audit?
- Tips to Make Your Auditing Smooth
- Pharmaceutical Audit Management Software Solution
What Is a Pharmaceutical Quality Audit?
Pharmaceutical quality audit is the systemized examination to check if your pharmaceutical organization follows the processes and procedures which are compliant to set standards. It also examines if the pharma company is producing products which match the set quality standards.
These quality audits also give feedback to the organization and find out the scope of improvement in process, procedures, or quality of the product to meet the standard of the market.
The entire motive of any pharmaceutical quality audit is to verify if the products being manufactured by the organization are safe to use for consumers or not.
Types of Audits in the Pharmaceutical Industry
Pharmaceutical industry will have three types of audits:
- Internal audit
- External audit
- Unannounced audit
Internal Audit – as an organization you audit your facilities, SOPs, other procedures frequently. Internal audits help to keep the day-to-day work and procedures smooth and without flaw.
With the help of an audit management system, like QualityPro’s QMS software for pharmaceutical industry, you can plan internal audits by managing audit schedules, creating regular assignments, and sending out notification to the right set of people.
External Audit– External audits are conducted by external parties. It can involve independent organization, like notified bodies and authorities. With the help of a pharmaceutical audit management system like QualityPro, organizations can easily conduct external audits, make reports, and send recommendations, if any, for further improvement.
Unannounced audits– Unannounced audits can be conducted randomly by organizations or third parties. The more complex is your manufacturing product, the more frequent can be such unannounced audits.
These are generally conducted to ensure that standard conduct and procedure following is in the DNA of the organization and it is not induced due to any audit requirement.
Ways in Which Pharmaceutical Audits are Conducted
There are three ways in which pharmaceutical audits are conducted:
- On-site audit
- Remote audit
- Self-audit
On-site audit– It is the audit in which third party auditor visits your organization and reviews documents, procedures, and processes. It will interview your staff, will see facilities and check if your organization meets the regulatory requirements.
Remote audit– Remote audit is very similar to on-site audit but with an exception. Auditor does not visit the site physically but visits the site virtually and connects with the organization via video conference. There has been a surge in remote- auditing post and during Covid due to various physical restriction. Like all other industries, audit also went remote, and this practice has become very commonly acceptable now.
Self-audit– Self-audit is the audit conducted by a department personnel to check if the department functions as per the set standards of quality, adheres to various laws, and functions according to SOPs.
Generally, these personnels are department heads who do self-audit. Self-audits are generally conducted frequently to make sure that all boxes of quality parameters are checked.
How to Prepare for Pharmaceutical Quality Audit?
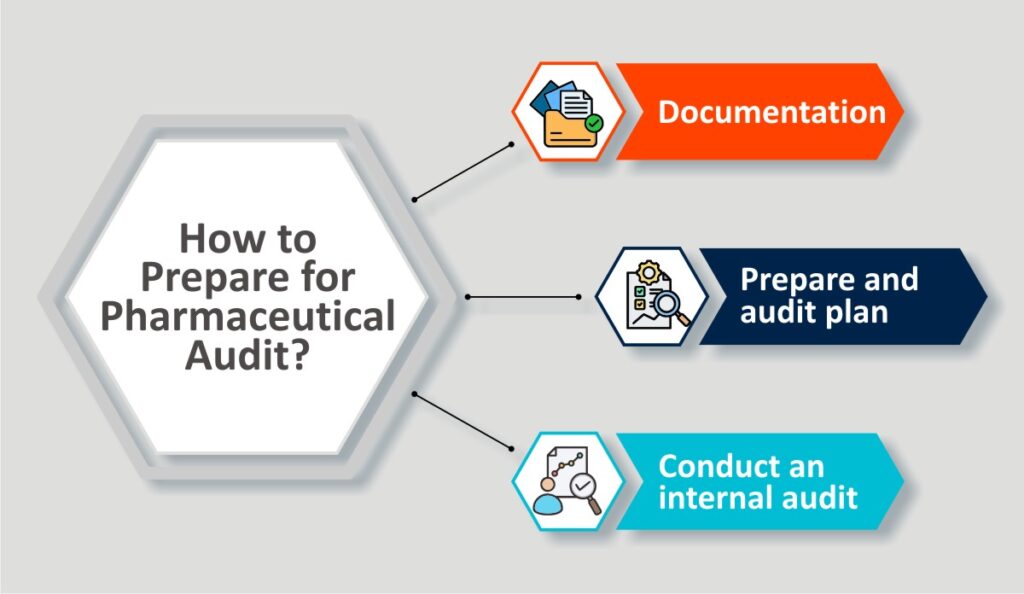
Preparation to audits can avert various chaos at eleventh hour. Hence, it is always advisable to be prepared for it. Here is some check list for smooth audit preparation.
Documentation–
Review all the past audits. Based on that, prepare a list of documents which consists of (but not limited to)
- Batch records
- Master formula records
- Standard operation protocols
- Change controls
- Deviation
These documents must be current and must be validated and arranged.
At such point of time, an efficient pharmaceutical document management system such as QualityPro comes into use. With the help of such a tool, you can easily arrange documents, create new documents, digitize documents, and make various versions (while keeping old versions).
Prepare an audit plan-
Prepare an audit plan and prepare a flow of the audit. This plan of action will be provided to audit inspector.
QualityPro by TecWork helps you to plan, define and document audit objectives, including the scope, purpose, and goals of the audit. It can also help to schedule different audits to execute in near future such as ISO and FDA as per your convenience.
Conduct an internal audit-
Internal audit will do a dry run before final audit. This will highlight any loophole in the process, procedure before the final audit. Remember to deploy an independent team for internal audit to make the audit fruitful.
Tips to Make Auditing Smooth:
- Make sure that all the key personnels are available at the time of audit.
- Provide a separate room for auditor.
- Be on time.
- Maintain professionalism.
- Take notes of auditing.
- Answer auditor’s all questions honestly.
- Provide only copies of documents and not originals.
- Don’t leave the auditor with your documents and notes.
- Conduct an exit interview with the auditor after the audit.
- Soon after the audit is completed, prepare for your answer for any discrepancy highlighted by the auditor.
Pharmaceutical Audit Management Software Solution:
Pharmaceutical companies depending upon old paper and pen-based model struggle and face lot of challenges regularly.
Recording on paper-based system is a very long and time-consuming process and generally it is error vulnerable. Documentation errors can lead to regulation non-compliance. This can be costly for the company in terms of money, brand image and time.
With the help of audit management software like QualityPro, organization can remove error-prone processes and digitize documentation recording process. It can offer tighter safety protocols, maintain audit trails, streamline audit workflow, and help organization to analyze findings and act proactively.
It offers an intuitive dashboard to get all audit related information like past audits, open findings, critical findings, CAPAs etc.
QualityPro for Pharmaceutical Industry Offers Numerous Benefits Like:
- It simplifies complete audit process
- Automates all time-consuming tasks saving time and resources
- Ensures compliance regulations
- Identifies potential risk and vulnerabilities
- Helps maintain integrity throughout the organization
- Pinpoint he grey areas in your organization that need improvement
To explore and discuss more on the benefits of QualityPro by TecWork for your pharmaceutical company or to explore how aptly does it fit to your unique requirement.





