
With Quality getting paramount importance everywhere, process manufacturing is no different. Terms like Quality Management System (QMS), Electronic Quality Management System (EQMS), and electronic Document Management System (EDMS) are gaining traction in board rooms, business meetings and on Google Search too. If you have been a part of such discussions, or have overheard or are wishing to know what these terms actually mean and function, then you are at the right place This blog elaborates their meanings, purpose and the distinguishing characteristics of them all for a better understanding. Continue reading-
What is a QMS?
A quality management system (QMS) is a formalized system that documents/describes the workflows, procedures, and responsibilities for achieving a company’s quality policies and objectives, the international standard specifying requirements for quality management systems is ISO 9001:2015 and it is one of the most widely used and prominent approach to quality management systems.
A QMS manages and coordinates an organization’s actions in order to conform to consumer and regulatory standards.
Also read: Different Types of Quality Management Systems
Who needs QMS Software?
In industries such as aviation, automotive, pharmaceuticals, food, and medical devices, where ensuring quality and safety is critical, the implementation of robust quality management systems (QMS) holds paramount importance.
For companies operating in these sectors, compliance with specific standards and regulations governing quality management is mandatory to legally sell their products.
Government agencies require these industries to adhere to stringent quality management system standards to ensure adherence to safety protocols and regulatory requirements.
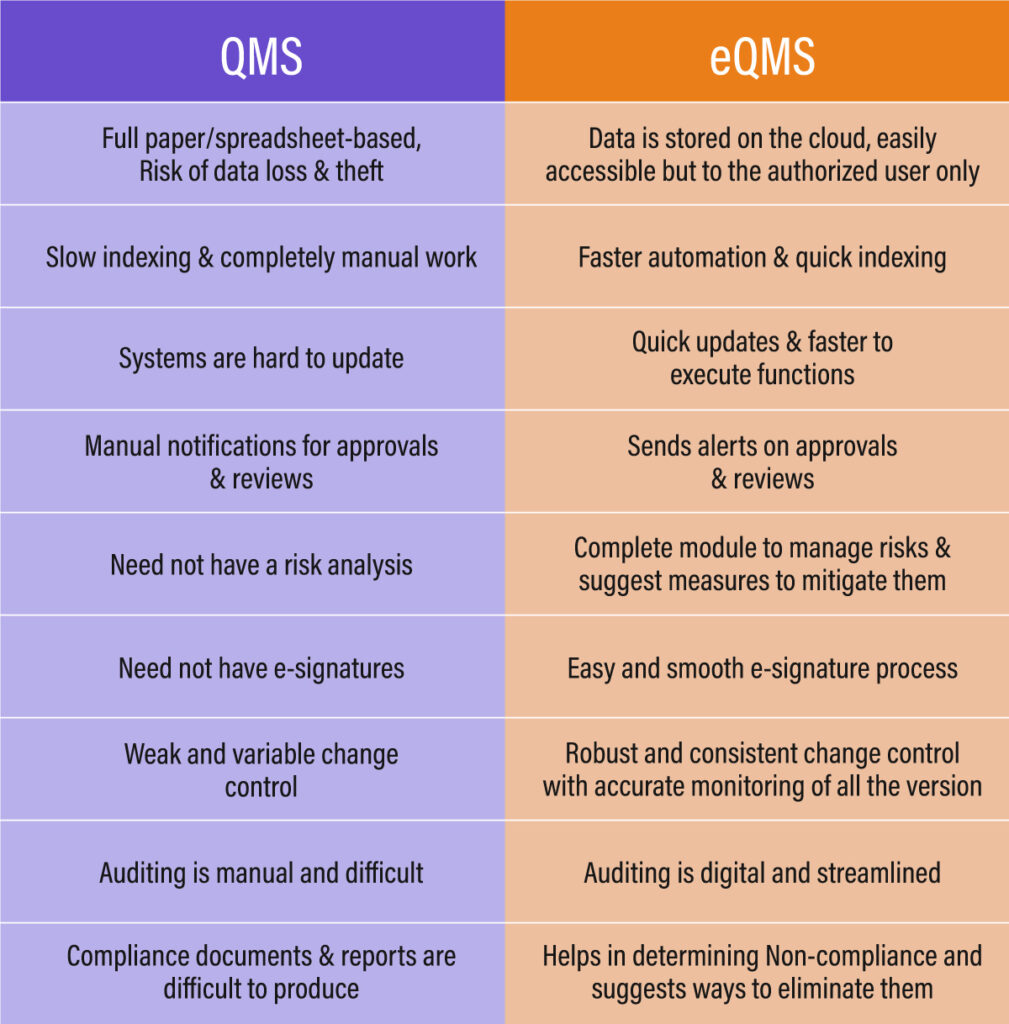
Traditional Paper-based QMS Software –
Managing documents through traditional paper-based methods has become outdated. The process demands considerable effort, time, and resources to keep data updated, often requiring workers to meticulously handle data entry tasks. Yet, despite their best efforts, there’s always a chance of missing something or making errors.
Manual file management is integral to various operations such as onboarding, record-keeping, investigations, and both internal and external audits. Even minor errors in the data can lead to significant miscalculations. Hence, there’s an urgent need to transition to an electronic data storage system.
This transition aims to minimize errors, boost productivity, and allocate resources towards tasks that significantly contribute to the overall efficiency.
What is eQMS Software?
A modernized approach to quality management systems is the electronic quality management system (eQMS). It revolutionizes the management and documentation of organizational-wide business processes like product development, quality assurance, and compliance, eliminating the need for manual paperwork.
The cloud-based version of eQMS operates on cloud servers and is accessible only by authorized users from any location and at any time. Essentially, eQMS encompasses all the components present in QMS solutions but with the added advantage of centralized accessibility and enhanced security.
eQMS has demonstrated superior performance over traditional QMS software due to the ease, efficiency, and flexibility it provides compared to conventional quality management systems.
What is EDMS Software?
An electronic document management system, or EDMS system, is a digital platform designed to manage various types of documents, whether they’re related to the Quality Management System, product development, HR, legal, or other categories. This system is versatile and can store different file formats like Microsoft Word, PDFs, 3D drawings, and more.
Traditionally, employees spent a considerable amount of time searching for and organizing documents manually, impacting their productivity in core tasks.
The transition from paper-based documentation to Electronic Document Management Systems has revolutionized accessibility, making documents available on smartphones or tablets.
EDMS solution isn’t limited to text documents; it accommodates various file types like images, videos, and audio files. It offers a unified view of all documents and incorporates features for printing, scanning, and storage.
A key advantage of an efficient EDMS software is its ability to automate document-related workflows, integrating seamlessly with other software such as ERP, QMS, or CRM systems. This connectivity streamlines processes and enhances overall operational efficiency.
This is how EDMS helps in building Quality Management –
To create a digital Quality management system, you can migrate all of your current quality documentation onto an EDMS, which is a cloud-based platform. It can benefit you with –
- Controlled document lifecycle: An EDMS streamlines document classification, organization, and archiving from creation to archival, enhancing searchability and indexing accuracy.
- Tailored workflows: Tailored for quality specialists, an EDMS offers pre-defined processes while allowing customization to suit unique operations, unlike an eQMS.
- Change and design control: Flexible adjustments to quality processes with comprehensive record-keeping for all modifications.
- Document review alerts: Ensures timely completion of review and approval cycles, maintaining the flow of documents within the system.
- Version control and audit trails: Automatically generates audit trails, displaying version histories, changes, and approvals for document transparency.
- Integration with e-signatures: Compliance-driven e-signature functionality (e.g., FDA 21 CFR part 11) to authorize documentations as per regulations.
- Enhanced SOPs and compliance records: Robust search and navigation features in an EDMS facilitate compliance inquiries, offering flexibility in SOP management while serving as a unified data source.
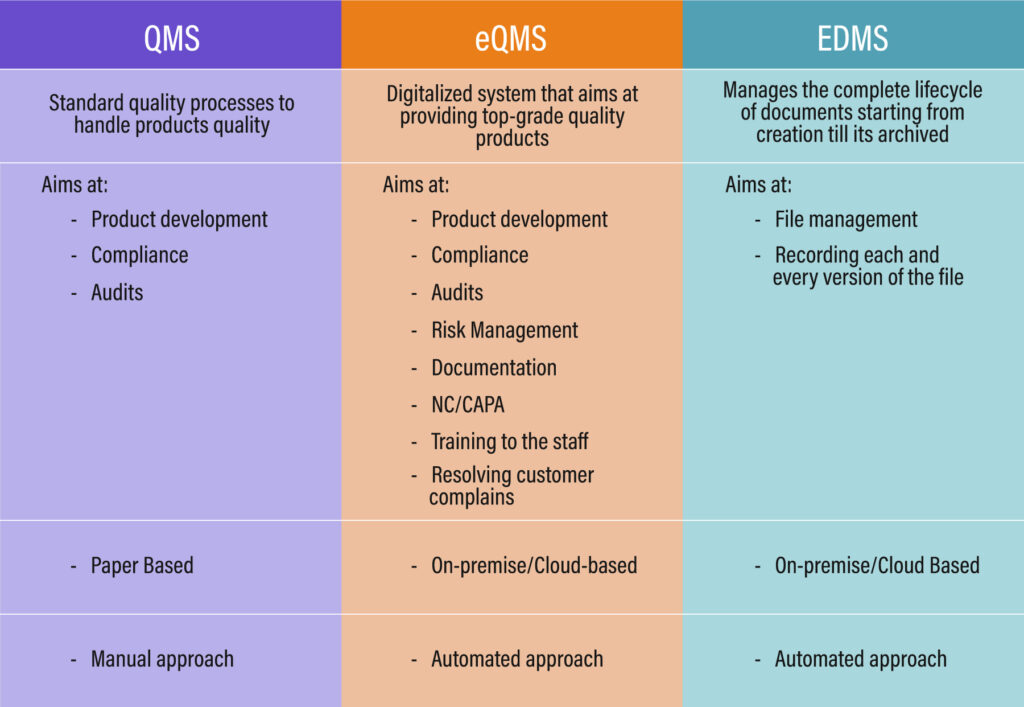
Difference between QMS, EQMS, and EDMS
While QMS solutions aim to meet quality procedures, they have grown obsolete and traditional; in contrast, eQMS has all the features built in accordance with technological advancements and rapidly shifting compliance standards. The line of distinction between these two names is extremely thin.
However, we may conclude that eQMS is a more contemporary and effective version of QMS solutions. The eQMS seeks to automate quality management operations, maintain compliance, monitor product quality, identify non-conformance, and conduct audit activities while reducing costs & time to market by keeping all of the important company information in a centralized repository.
When it comes to EDMS, it differs slightly from eQMS and QMS. The area that this solution covers completely is the documentation and its lifecycle from creation to storage, however, EDMS is also a part of handling quality and eQMS.
QMS, EQMS, or EDMS, which one do you need?
Choosing the right solutions out of these three depends on the type of need your business operations have. The choice becomes clear only when you know what your business requirements are, what your strategy is, and which solution fits the best within your business ecosystem.
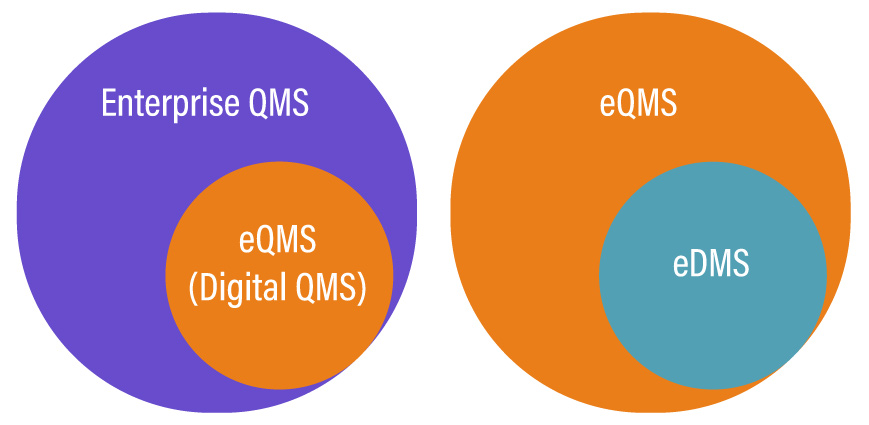
You can prefer an eQMS system as it is a complete solution serving the needs of a QMS and an EDMS. It outdoes the traditional paper-based quality management systems in ways uncountable and is quickly becoming the new standard for quality management across businesses operating in various industries and of different sizes.
If you’re also looking for an eQMS that can take your quality management process to the next level and instills a culture of Quality, QualityPro is the right solution for you. Contact our experts for details.





